

July Sale: Limited Time 20% Off!
Use the promo code J20 at checkout.


Success Rate

Up to 27% more effective than oral medicine
Published by Emergency Medicine in the United States
Learn More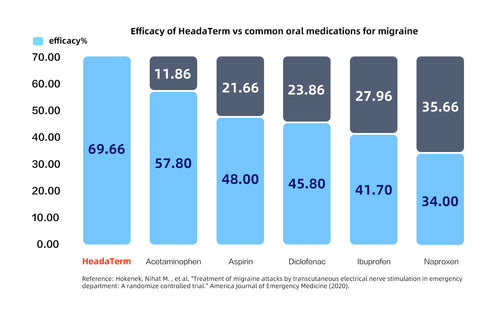
| Brand | HeadaTerm |
|---|---|
| Non-rechargeable | The device lasts 20 minutes each time and can be used for up to 7 hours. |
| Principle | eTNS technology |
| Designer | Canada |
| Indications | Prevent and treat primary headache, including migraine, tension headache, cluster headache, depression and insomnia. |
| Certificate | FDA, CE, TGA, HC, ISO |
HeadaTerm Anti-Migraine Device
HeadaTerm is a clinically proven, safe, and effective medical device worn on users’ foreheads that uses none-invasive neuronal electrical stimulation technology to prevent and treat primary headache, including migraine, tension headache, and cluster headache.
WHY EmeTerm® HeadaTerm Anti-migraine Device
Affordable Migraine Relief Products

Portable

100% Drug-Free

Easy Operation

Quick Effect
New Generation Of Targeted Thrapeutic Anti-Migraine Device
Primary headaches are debilitating conditions that are extremely common among people. Drug treatments are the popular solutions for many patients, but just like with anti-nausea drugs, there are side effects that often turn people away. HeadaTerm offers a drug-free treatment without any side effects.

Headache

Migraine

Insomnia

Depression
Adjustable Stimulation Intensity
- The intensity of the treatment will increase to the strongest level within five minutes.
- During this period, users can choose the appropriate intensity according to their own experience to achieve the best effect.


Compact And Easy To Carry
- HeadaTerm is very small and light, making it convenient not only to use at home or at the office, but also on trips and outdoor travelling.
How to use
HOW IT WORKS

Primary headaches, such as migraine, are transmitted by the supraorbital nerve and the supratrochlear nerve. HeadaTerm introduces precise electric impulses from the user’s forehead to act on these nerves and reduce the migraine signals transmitted. The device avoids any drug effects, making it appropriate a wide range of users. Every device contains 21 rounds of standard therapies, each therapy lasts 20 minutes.

HeadaTerm Official Sustainability Plan
Every 5 used devices can be exchanged for 1 new device.
Learn MoreFAQ
EmeTerm® HeadaTerm Testimonials
Customers Testimonials
Reference
- Hokenek N M, Erdogan M O, Hokenek U D, et al. Treatment of migraine attacks by transcutaneous electrical nerve stimulation in emergency department: A randomize controlled trial[J]. The American Journal of Emergency Medicine, 2021, 39: 80-85.
- Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. J Pain. 2003 Apr;4(3):109-21.
- Shealy CN. Transcutaneous electrical nerve stimulation: the treatment of choice for pain and depression. J Altern Complement Med. 2003 Oct;9(5):619-23.
- Huang W, Kutner N, Bliwise DL. Autonomic activation in insomnia: the case for acupuncture. J Clin Sleep Med. 2011 Feb 15;7(1):95-102.
- Kirsch DL, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013 Mar;36(1):169-76.
- Schoenen J, Vandersmissen B, Jeangette S, et al. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology. 2013 Feb 19;80(8):697-704.
- Nihat M. Hokenek, MD. Treatment of migraine attacks by transcutaneous electrical nerve stimulation in emergency department: A randomized controlled trial. VOLUME 39, P80-85, JANUARY 01, 2021














