




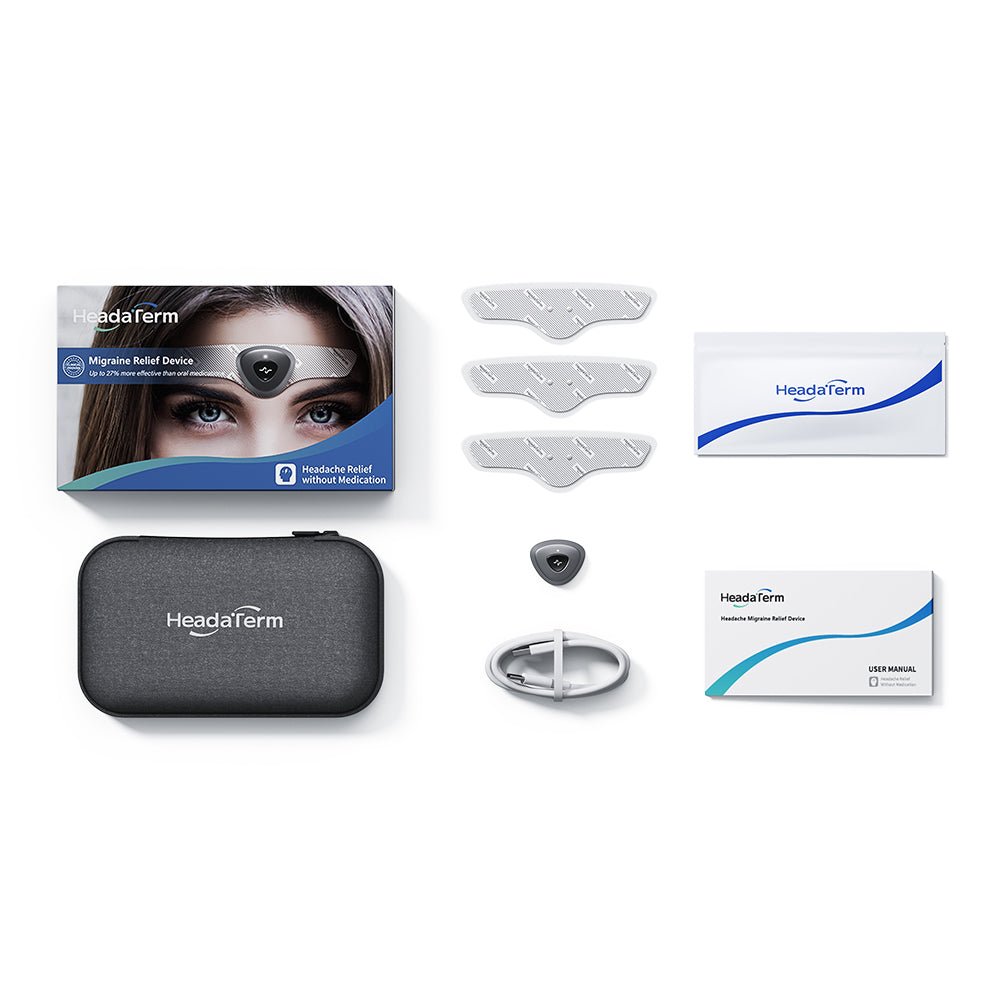


Exclusive Early Bird Price for the HeadaTerm2
Act fast! Only 30 early bird slots available – reserve yours now!
Discount Code: PREHT25

Safety Certification
FDA-approved for safety and effectiveness.

Physical Therapy
Fast-acting relief with eTNS technology and no side effects.

Non-prescription
Affordable home medical products.

HSA/FSA Eligible
Vouchers available for reimbursement upon purchase.

Up to 35% more effective than oral medicine
Published by Emergency Medicine in the United States
Learn More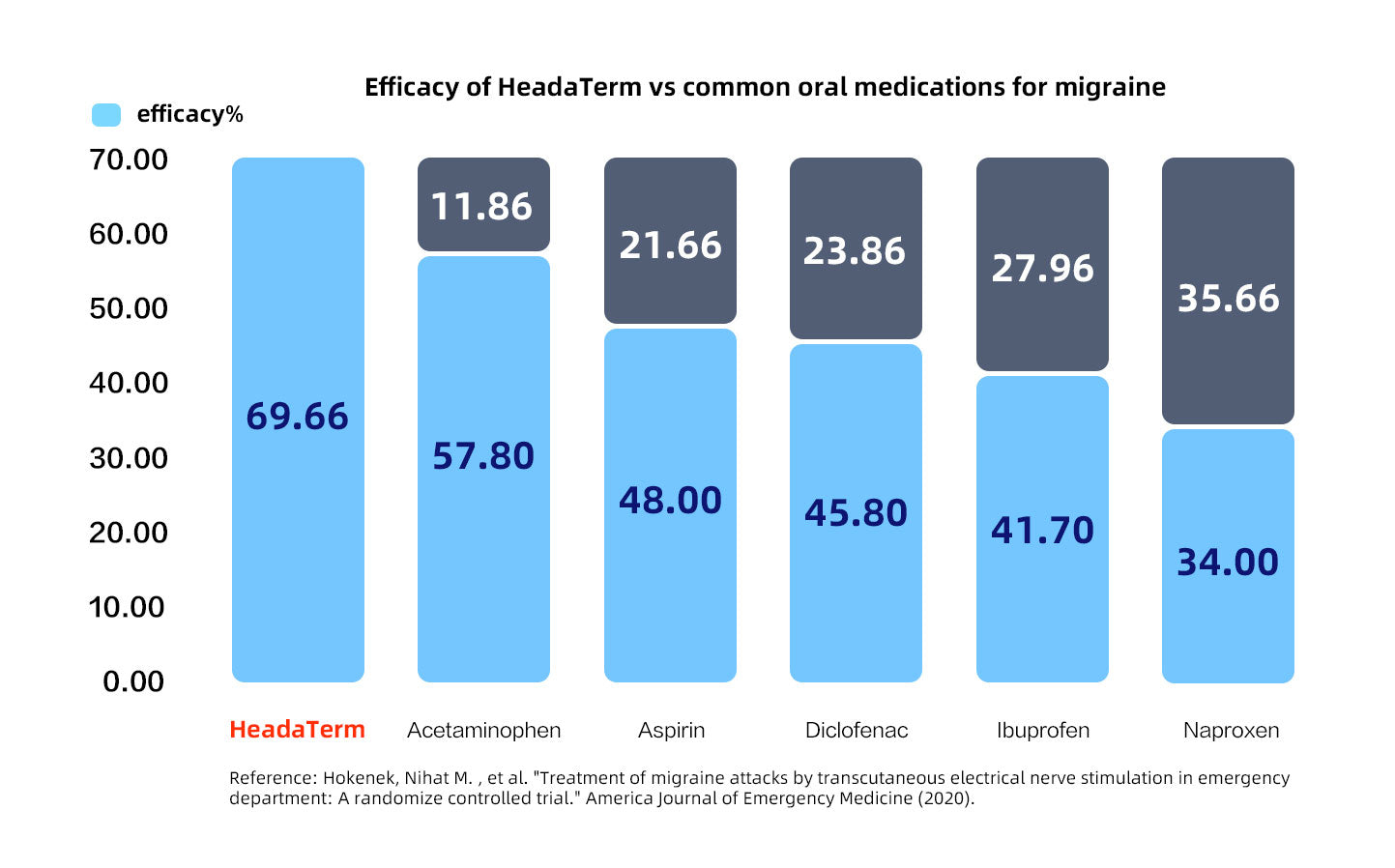
HOW IT WORKS


Primary headaches, such as migraine, are transmitted by the supraorbital nerve and the supratrochlear nerve. HeadaTerm introduces precise electric impulses from the user’s forehead to act on these nerves and reduce the migraine signals transmitted. The device avoids any drug effects, making it appropriate a wide range of users.
Three Major Benefits of HeadaTerm 2


Treating Acute Migraine Attacks
58%
Effective in Significantly Relieving Acute Migraine Symptoms

Reducing Migraine Severity
86%
Effective in Reducing Migraine Pain Levels

Preventing Future Migraines
64%
Effective in Reducing the Incidence of Migraines

For every HeadaTerm 2 Anti-migraine Device purchased, $1 will be donated to The American Legion.
*Donate every 5 months and publish the donation voucher
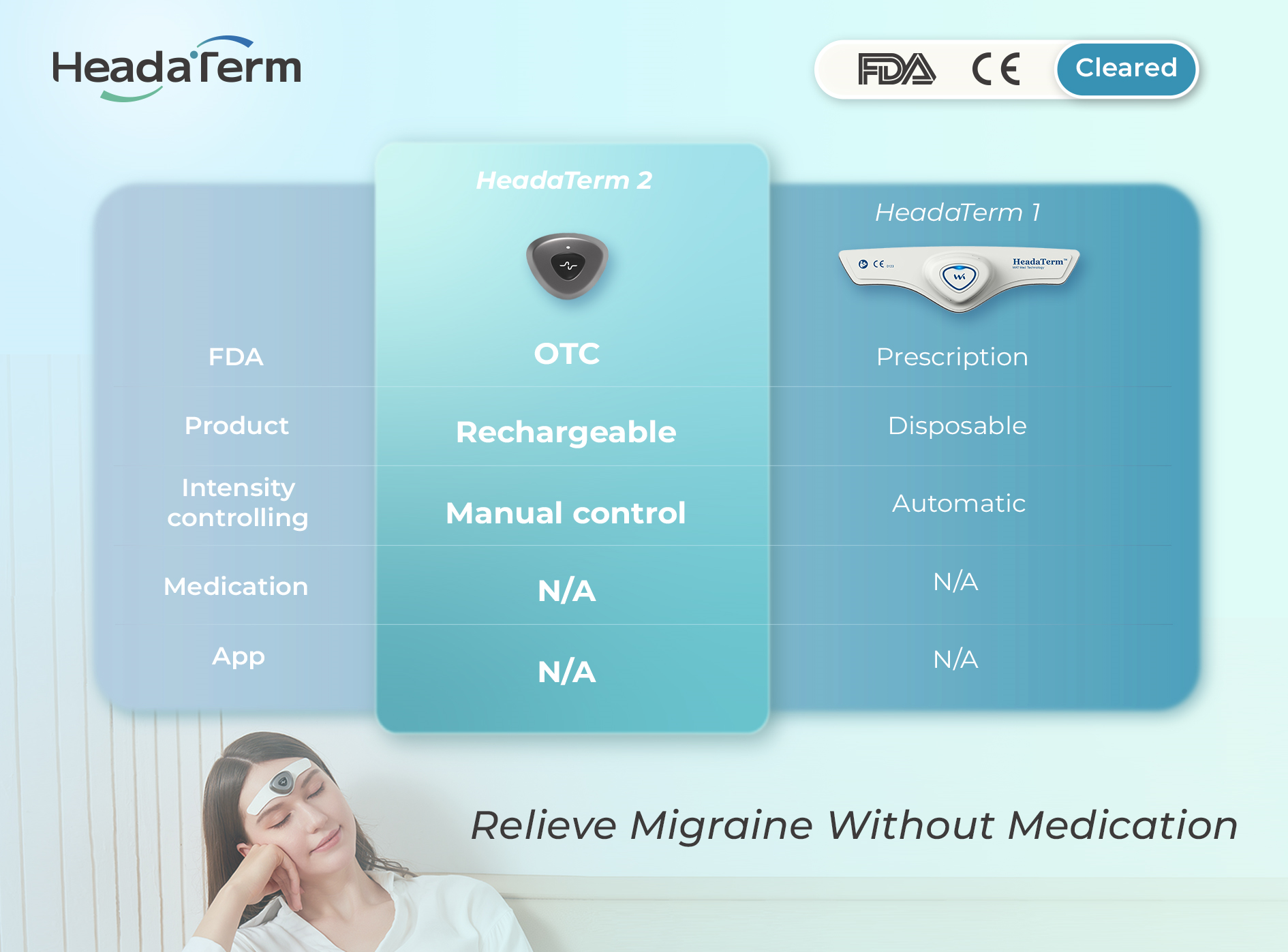
Learn more| Brand | HeadaTerm |
|---|---|
| Rechargeable | |
| Principle | eTNS technology |
| Designer | Canada |
| Indications | HeadaTerm 2 is indicated for the prophylactic treatment of episodic migraine in patients 18 years of age or older. |
| Certificate | FDA, CE, TGA, HC, ISO |

Portable

100% Drug-Free

Easy Operation

Quick Effect
New Generation Of Targeted Thrapeutic Anti-Migraine Device
Primary headaches are debilitating conditions that are extremely common among people. Drug treatments are the popular solutions for many patients, but just like with anti-migraine drugs, there are side effects that often turn people away. HeadaTerm offers a drug-free treatment without any side effects.
More advanced than HeadaTerm Generation 1.
How to use
Learn more
HeadaTerm 2 Electrode (3-pack)

HeadaTerm Official Sustainability Plan
Every 5 used devices can be exchanged for 1 new device.
Learn MoreEmeTerm® HeadaTerm Testimonials
Customers Testimonials
Reference
- Hokenek N M, Erdogan M O, Hokenek U D, et al. Treatment of migraine attacks by transcutaneous electrical nerve stimulation in emergency department: A randomize controlled trial[J]. The American Journal of Emergency Medicine, 2021, 39: 80-85.
- Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. J Pain. 2003 Apr;4(3):109-21.
- Shealy CN. Transcutaneous electrical nerve stimulation: the treatment of choice for pain and depression. J Altern Complement Med. 2003 Oct;9(5):619-23.
- Huang W, Kutner N, Bliwise DL. Autonomic activation in insomnia: the case for acupuncture. J Clin Sleep Med. 2011 Feb 15;7(1):95-102.
- Kirsch DL, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013 Mar;36(1):169-76.
- Schoenen J, Vandersmissen B, Jeangette S, et al. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology. 2013 Feb 19;80(8):697-704.
- Nihat M. Hokenek, MD. Treatment of migraine attacks by transcutaneous electrical nerve stimulation in emergency department: A randomized controlled trial. VOLUME 39, P80-85, JANUARY 01, 2021



















